Dissolving Calcium Chloride in Water Physical or Chemical
Wash thoroughly after handling. Dissolving feeders regulate the rate at which a dry chemical is dissolved.

Calcium Chloride Cas No 10043 52 4 Latest Price Manufacturers Suppliers
The chemical reaction between sodium alginate and calcium chloride to form calcium alginate was utilized for microsphere formation.
. Sugar dissolves in water but it doesnt break into any ions. A substance that accepts an H from another substance. Sodium chloride breaks into two ions in water.
Evaporation of the sea water is one of the major processes used to obtain salt and is most widely followed in countries like India. It is a white solid ionic compound consisting of sodium cations Na and hydroxide anions OH. The solution was stirred with a sterile glass rod for complete mixing.
Calcium hypochlorite is the solid form of chlorine used as a disinfectant. A number of factors are involved in the extent to which lead enters the water including. By dissolving water and making it harder to freeze it decreases ice buildup and serves as a deicer.
The metal is trimorphic harder than sodium but softer than aluminiumA well as beryllium and aluminium and unlike the alkaline metals it doesnt cause skin-burns. Sodium alginate solution 4 was prepared by dissolving sodium alginate in distilled water kept in a water bath at 6575 C. Store capped at.
A dissolving tank is charged with dry chemical and a regulated flow of water is fed into the vessel. The benefits of calcium hypochlorite are that it is more stable than the liquid form and it has a longer shelf life. In this lab you will.
It is less chemically reactive than alkaline metals and than the other alkaline. CHEM 1100 3 Equation 2. The concentration of discharged product is governed by the contact area between the dry material and water and the rate of dissolution.
Next carbonic acid reacts with minerals in rocks to produce carbonates or bicarbonates. Determine the molarity of a solution that is made by dissolving 455 g of NaNO3 in sufficient. Calcium chloride forms three ions in water.
Do not ingest or inhale. First carbon-dioxide reacts with water to form carbonic acid. Do not get on skin or in eyes.
Calcium chloride ACS - 3 - Section 7 - Handling and Storage Handling. How might you use exothermic or endothermic processes to solve a real-world problem. This is a common practice in swimming pools or anywhere that concrete holds water.
Water is a liquid at ambient conditions but it often co-exists on Earth with its solid state ice and gaseous state water vapor or steam. The crystals obtained usually consists of impurities such as calcium sulfate sodium sulfate etc. The chemical element Calcium Ca atomic number 20 is the fifth element and the third most abundant metal in the earths crust.
Manganese sulphide formed in the group analysis dissolves in dilHCl forming manganese chloride and H 2 S is boiled off. Its molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. H 2 C O C O R CH O C R H 2 C O O C O R 1.
3 mL distilled water 5 drops 3 calcium chloride solution. See if you can dissolve equal masses of each substance in the water in order to get a fair comparison. Sodium hydroxide is a highly caustic base and alkali that decomposes proteins at ordinary ambient temperatures and may cause severe chemical burnsIt is highly.
Calcium hydroxide traditionally called slaked lime is an inorganic compound with the chemical formula Ca 2It is a colorless crystal or white powder and is produced when quicklime calcium oxide is mixed or slaked with waterIt has many names including hydrated lime caustic lime builders lime slaked lime cal and pickling limeCalcium hydroxide is used in many. Use with adequate ventilation. Adding calcium chloride to soft water makes it harder.
Heat evolved is significant. The primary drawback in using solid chlorine is that. Avoid breathing dust vapor mist or gas.
Pure crystals are obtained by dissolving the salts with little water and filtering the solution. All of these substances will lower the freezing point of water. A substance that is capable of neutralizing an acid in a chemical reaction forming water.
The chemistry of the water acidity and alkalinity and the types and amounts of minerals in the water. For example physical and chemical properties exposure control and toxicology data. A Sodium hydroxide - bromine water test.
PHYSICAL AND CHEMICAL PROPERTIES OF WATER Water is a chemical substance with the chemical formula H2O. Corrosion is a dissolving or wearing away of metal caused by a chemical reaction between water and your plumbing. The manganese chloride formed by dissolving MnS in HCl reacts with excess of NaOH to form white precipitate of manganese hydroxide.
Using the language of breaking and making bonds how can you describe the temperature change you observed when you dissolved calcium chloride in water. As with all other chemicals used in water treatment there are benefits and drawbacks to its use. Carbonation of rocks containing calcium carbonate limestone is a common process of chemical weathering which leads to the formation of calcium bicarbonate that is highly soluble in water.
Always use cool water when dissolving calcium chloride. Describe the ratio if calcium to chloride ions in the ionic compound calcium chloride CaCl2. 1 synthesis soap and ii study the physical and chemical properties of your soap.
Sodium hydroxide also known as lye and caustic soda is an inorganic compound with the formula NaOH. The polar head is capable of dissolving polar molecules such as water. Is dissolving calcium chloride in water a chemical change.
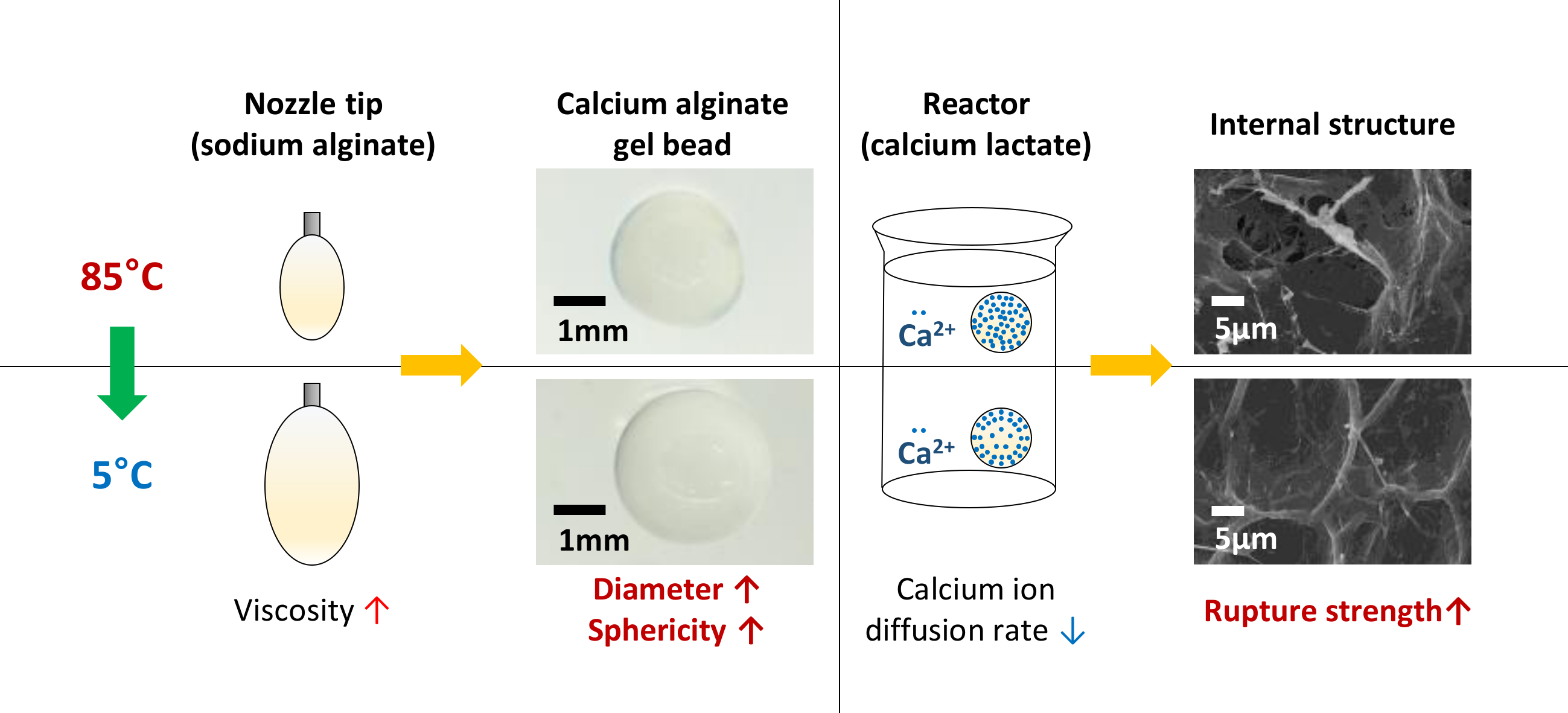
Foods Free Full Text Changes In The Physical Properties Of Calcium Alginate Gel Beads Under A Wide Range Of Gelation Temperature Conditions Html
How Does The Water Become Hard On Adding A Sufficient Amount Of Calcium Chloride Or Magnesium Chloride Quora

Calcium Chloride An Overview Sciencedirect Topics
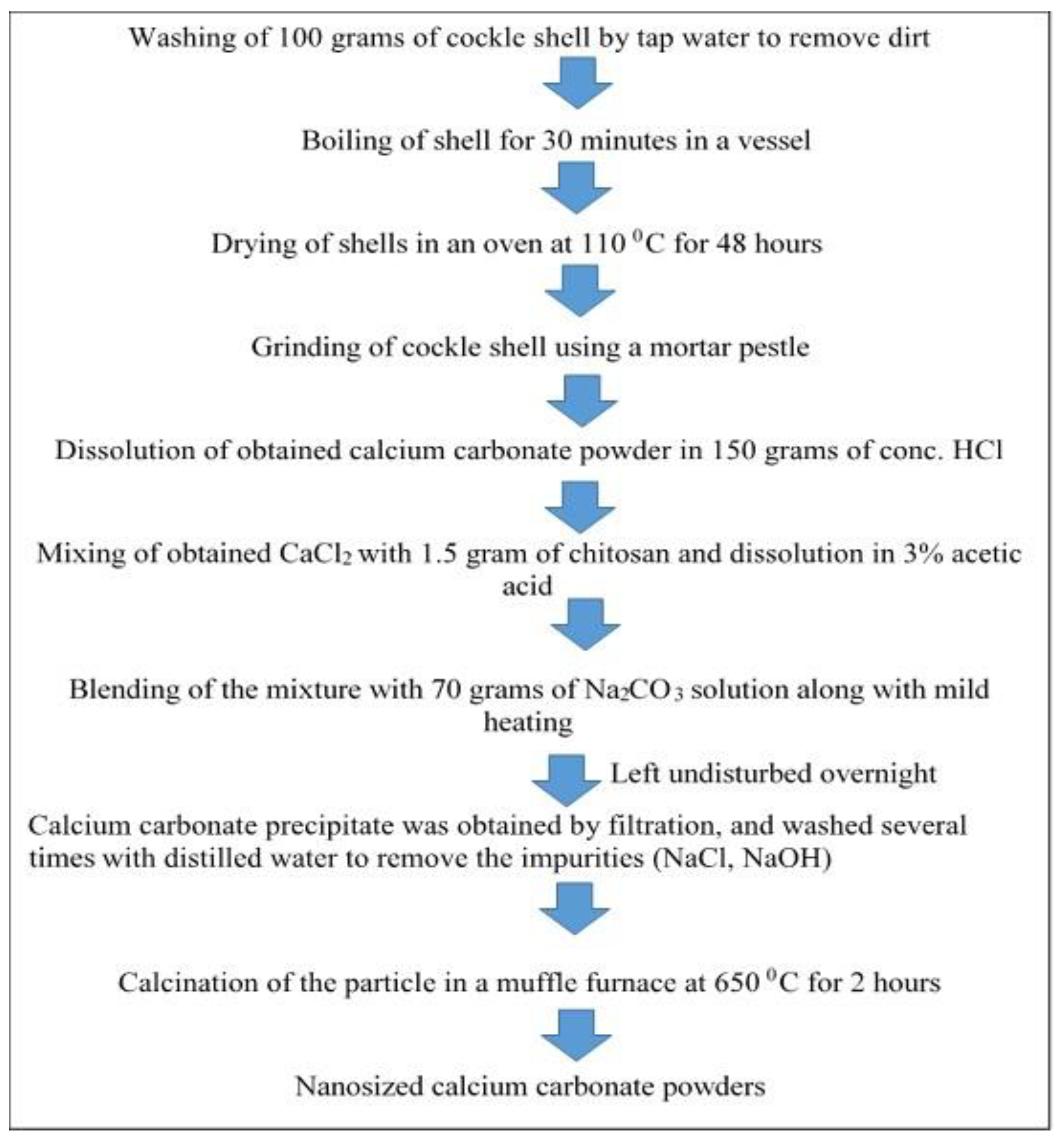
Applied Sciences Free Full Text The Processing Of Calcium Rich Agricultural And Industrial Waste For Recovery Of Calcium Carbonate And Calcium Oxide And Their Application For Environmental Cleanup A Review Html

Calcium Chloride An Overview Sciencedirect Topics

Cacl2 H2o Calcium Chloride Water Youtube

Clues To Chemical Changes With Examples Significant Release Of Heat Reaction Of Calcium Chloride And Water Cacl 2 H 2 O Ca Oh 2 2hcl Ppt Download

Calcium Chloride Hazards Formula And Uses Free Chemistry Online
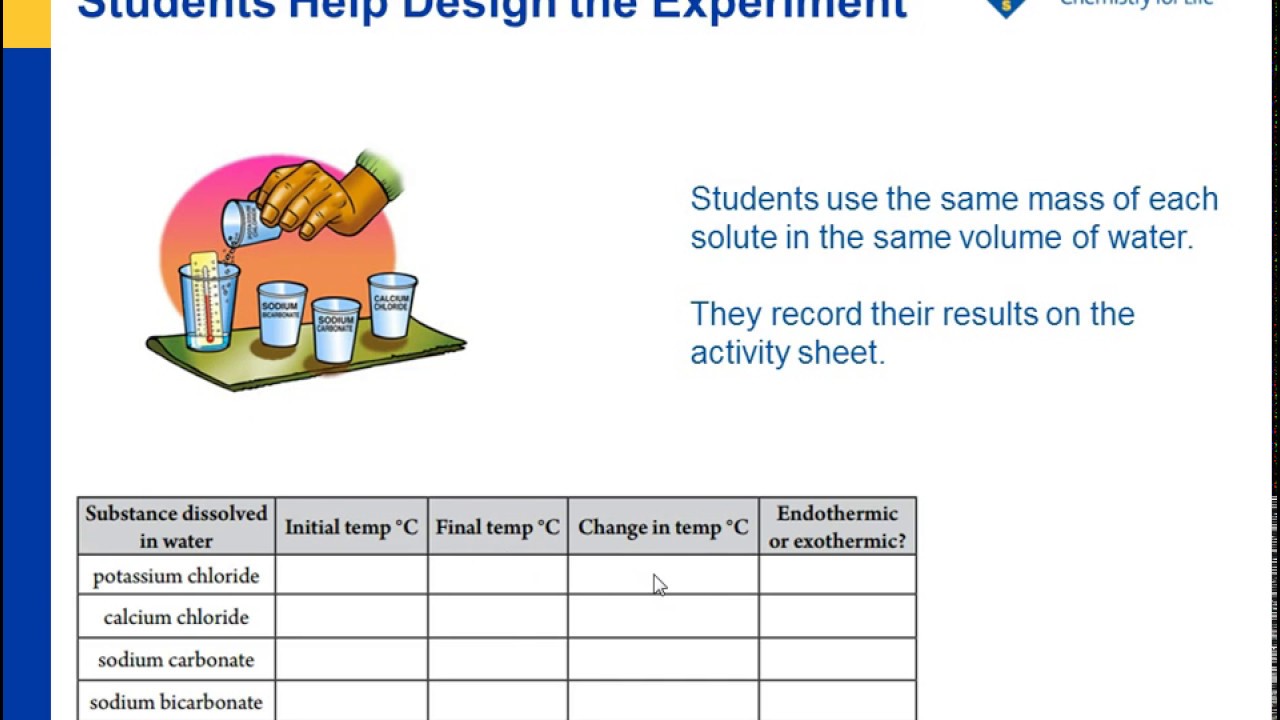
Temperature Changes In Dissolving Chapter 5 The Water Molecule And Dissolving Middle School Chemistry
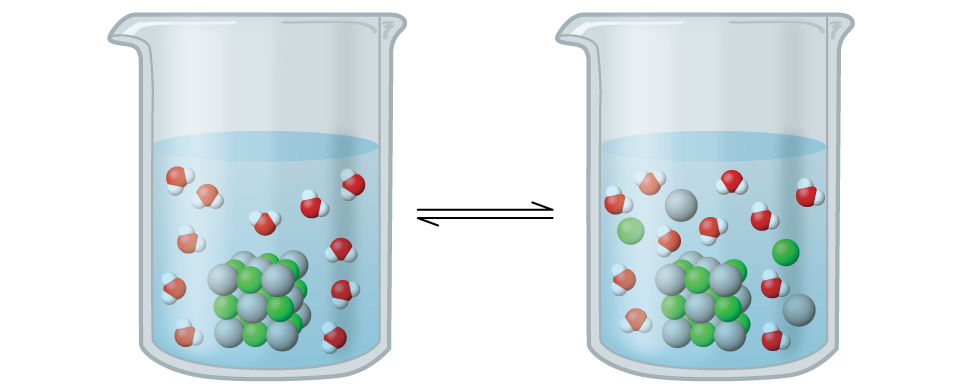
15 1 Precipitation And Dissolution Chemistry

Calcium Chloride An Overview Sciencedirect Topics

What Type Of Reaction Would Be Expected When Sodium Phosphate Reacts With Calcium Chloride Quora
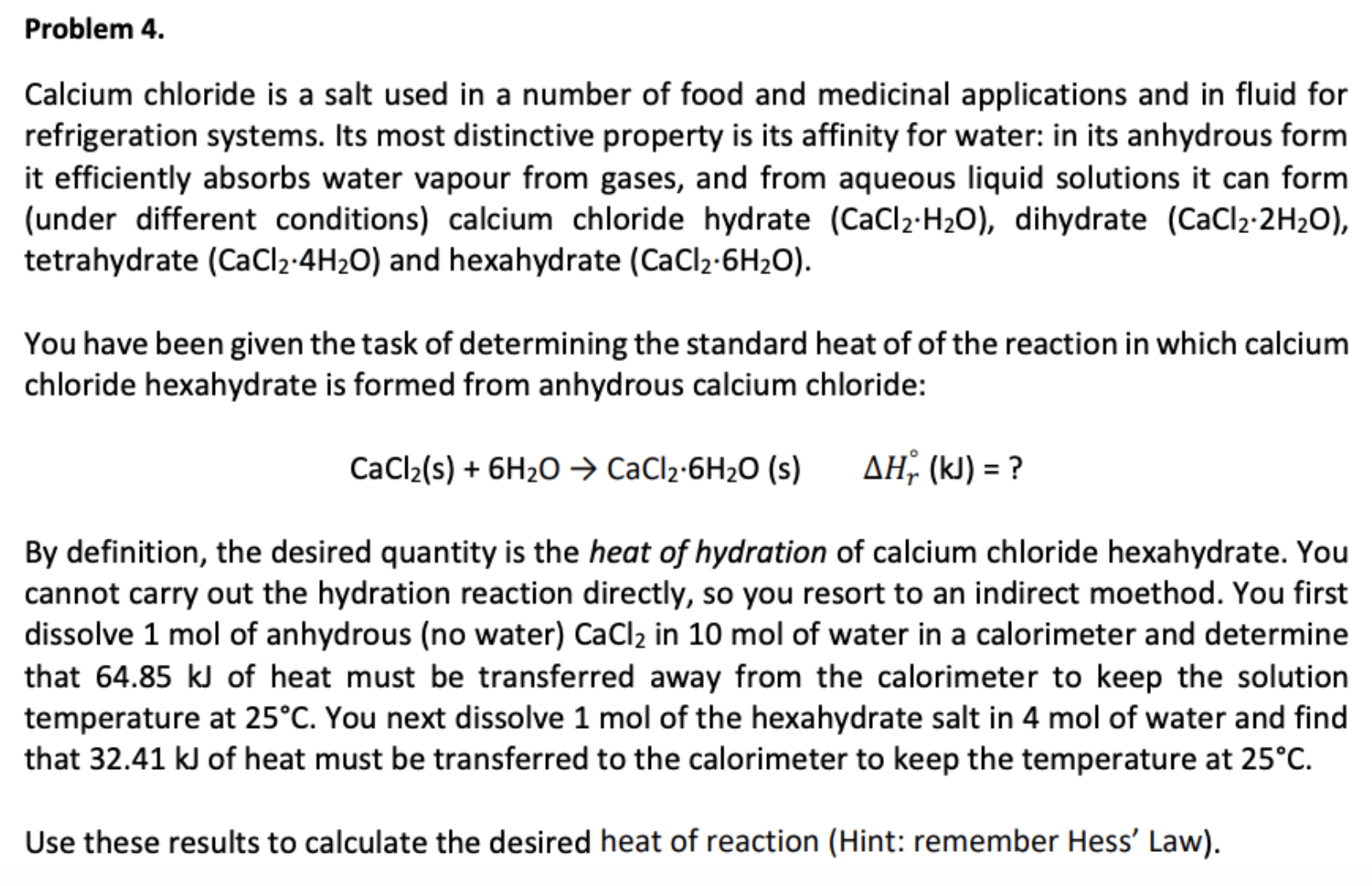
Answered Problem 4 Calcium Chloride Is A Salt Bartleby
Calcium Chloride Cacl2 Structure Properties And Uses

Which Figure Below Best Represents An Aqueous Solution Of Cacl2 Ppt Download

Read Each Statement And Identify It As An Example Of A Physical Or Chemical Change 1 Chopping Wood 2 Dissolving Salt In Water 3 Cooking Egg White 4 A Ppt Download

Is Calcium Chloride Soluble In Water

Question Video Writing A Net Ionic Equation For The Reaction Of Solid Calcium Carbonate With A Hydrochloric Acid Solution Nagwa
Comments
Post a Comment